Disease⁚ MNGIE Syndrome
Disease Overview

Mitochondrial neurogastrointestinal encephalopathy (MNGIE) syndrome is a rare genetic disorder that primarily affects the digestive and nervous systems. Individuals with MNGIE syndrome often experience gastrointestinal dysmotility, neurological symptoms, and muscle weakness. This condition typically emerges in the first or second decade of life and worsens over time. MNGIE syndrome is caused by mutations in the TYMP gene, leading to thymidine phosphorylase deficiency and mitochondrial DNA abnormalities. Common symptoms include early satiety, nausea, vomiting, diarrhea, ophthalmoplegia, and peripheral neuropathy. Diagnosis is based on clinical presentation, genetic testing, and imaging studies. While there is no cure for MNGIE syndrome, treatment focuses on managing symptoms, such as nutritional support and enzyme replacement therapy. Research into potential therapies, such as stem cell transplantation, is ongoing to improve outcomes for individuals affected by this debilitating syndrome.
Disease Name and Synonyms
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), also known as mitochondrial neurogastrointestinal encephalopathy, is a rare genetic disorder caused by mutations in the TYMP gene. This condition is sometimes referred to as mitochondrial neurogastrointestinal encephalomyopathy syndrome or MNGIE disease. Individuals with MNGIE syndrome may also hear terminologies like mitochondrial neurogastrointestinal encephalopathy disease or mitochondrial neurogastrointestinal encephalomyopathy syndrome.
Introduction
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare autosomal recessive disorder caused by mutations in the TYMP gene, leading to thymidine phosphorylase deficiency and mitochondrial DNA abnormalities. This results in severe gastrointestinal dysmotility and neurological manifestations. The condition typically emerges in the first or second decade of life and progresses over time with debilitating symptoms affecting various body systems. Understanding the underlying genetic basis and clinical presentation of MNGIE is crucial for accurate diagnosis and management of affected individuals.
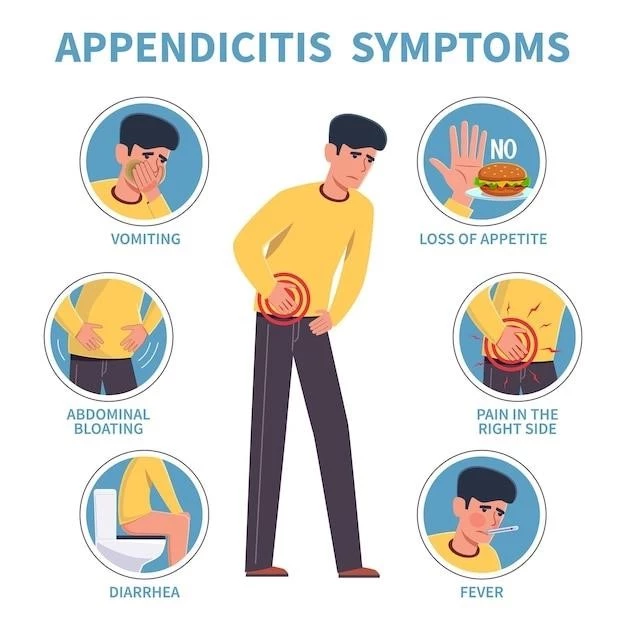
Symptoms and Clinical Features
MNGIE syndrome presents a spectrum of symptoms affecting the gastrointestinal system, nervous system, and muscles. Common indications include gastrointestinal dysmotility leading to early satiety, nausea, vomiting, and diarrhea. Neurological symptoms such as ophthalmoplegia, peripheral neuropathy, and muscle weakness are prevalent. Individuals may also experience weight loss, weakness, and fatigue. Severe cases can exhibit leukoencephalopathy, ptosis, and cachexia. The progression of MNGIE syndrome can result in a significant impact on the overall quality of life for affected individuals.
Causes of MNGIE Syndrome
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome is primarily caused by mutations in the TYMP gene, leading to thymidine phosphorylase deficiency. This deficiency results in abnormalities in mitochondrial DNA replication, including depletion, deletions, and point mutations, affecting mitochondrial function. The accumulation of thymidine and 2-deoxyuridine contributes to mitochondrial failure and the manifestation of gastrointestinal and neurological symptoms observed in individuals with MNGIE syndrome.
Genetic Inheritance
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome follows an autosomal recessive inheritance pattern, with mutations in the TYMP gene on chromosome 22q13.32-qter being responsible for the condition. Individuals inherit one mutated gene from each parent٫ resulting in a total abolition of thymidine phosphorylase enzyme activity. This enzymatic deficiency leads to the accumulation of thymidine and deoxyuridine in body fluids and tissues٫ causing mitochondrial dysfunction and impacting mitochondrial DNA replication.
Diagnosis and Testing
Diagnosing mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome involves a combination of clinical evaluation, genetic testing, and imaging studies. Medical professionals may perform blood tests to assess thymidine and deoxyuridine levels, muscle biopsies to evaluate mitochondrial DNA abnormalities, and imaging scans to identify neurological and gastrointestinal complications. Genetic testing plays a crucial role in confirming mutations in the TYMP gene, establishing a definitive diagnosis of MNGIE syndrome.
Treatment Approaches
Management of mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome involves a multidisciplinary approach to address the diverse symptoms impacting the gastrointestinal and neurological systems. Current treatment strategies focus on symptom management and supportive care, including nutritional support to combat weight loss and enzyme replacement therapy to address deficiencies. Emerging therapies such as allogeneic stem cell transplantation and platelet transfusions are being explored to potentially alter the disease course and improve patient outcomes. Regular monitoring and individualized care plans are essential in the comprehensive management of individuals with MNGIE syndrome.
Management Strategies
Management of mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome requires a comprehensive approach focusing on addressing the diverse symptoms affecting the gastrointestinal and neurological systems. Treatment strategies typically involve a combination of supportive care, including nutritional support to combat weight loss and enzyme replacement therapy for associated deficiencies. Emerging therapies such as allogeneic stem cell transplantation and platelet transfusions are being explored to potentially alter the disease course and improve patient outcomes. Regular monitoring and individualized care plans tailored to the unique needs of each patient are essential components of successful management.

Impact on Body Systems
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome significantly impacts various body systems, particularly the gastrointestinal and neurological systems. The condition’s manifestations include severe gastrointestinal dysmotility leading to symptoms like early satiety, nausea, vomiting, and diarrhea. Neurological complications such as ophthalmoplegia, peripheral neuropathy, and muscle weakness also contribute to the systemic impact of MNGIE syndrome. These ongoing challenges affect the overall well-being and quality of life of individuals living with this rare genetic disorder.
Prognosis and Life Expectancy
The prognosis of individuals with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome varies based on the severity of symptoms and the timing of diagnosis. Early detection and comprehensive management can potentially improve outcomes and quality of life for affected individuals. However, MNGIE syndrome is a progressive disorder that often leads to significant morbidity and can ultimately impact life expectancy. Close monitoring, timely interventions, and ongoing research efforts are essential in enhancing the prognosis and addressing the complex challenges associated with this rare genetic condition.
Research and Advancements
Research into mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome is actively advancing, focusing on understanding the underlying genetic mechanisms and developing novel treatment approaches. Recent studies have explored potential therapies such as allogeneic stem cell transplantation and platelet transfusions to address mitochondrial dysfunction in affected individuals. Additionally, ongoing research seeks to elucidate the complex interactions between TYMP mutations, thymidine phosphorylase deficiency, and mitochondrial DNA abnormalities, aiming to improve diagnostics and therapeutic interventions for individuals with MNGIE syndrome.
Conclusion
In conclusion, mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome poses a significant challenge due to its progressive nature and impact on multiple body systems. While advancements in research offer promise for potential treatments such as stem cell transplantation and enzyme replacement therapy, the complexity of this rare genetic disorder necessitates ongoing efforts to improve diagnostics and therapeutic interventions. Collaborative initiatives between clinicians, researchers, and affected individuals are essential to enhance our understanding of MNGIE syndrome and ultimately improve outcomes for those living with this debilitating condition.